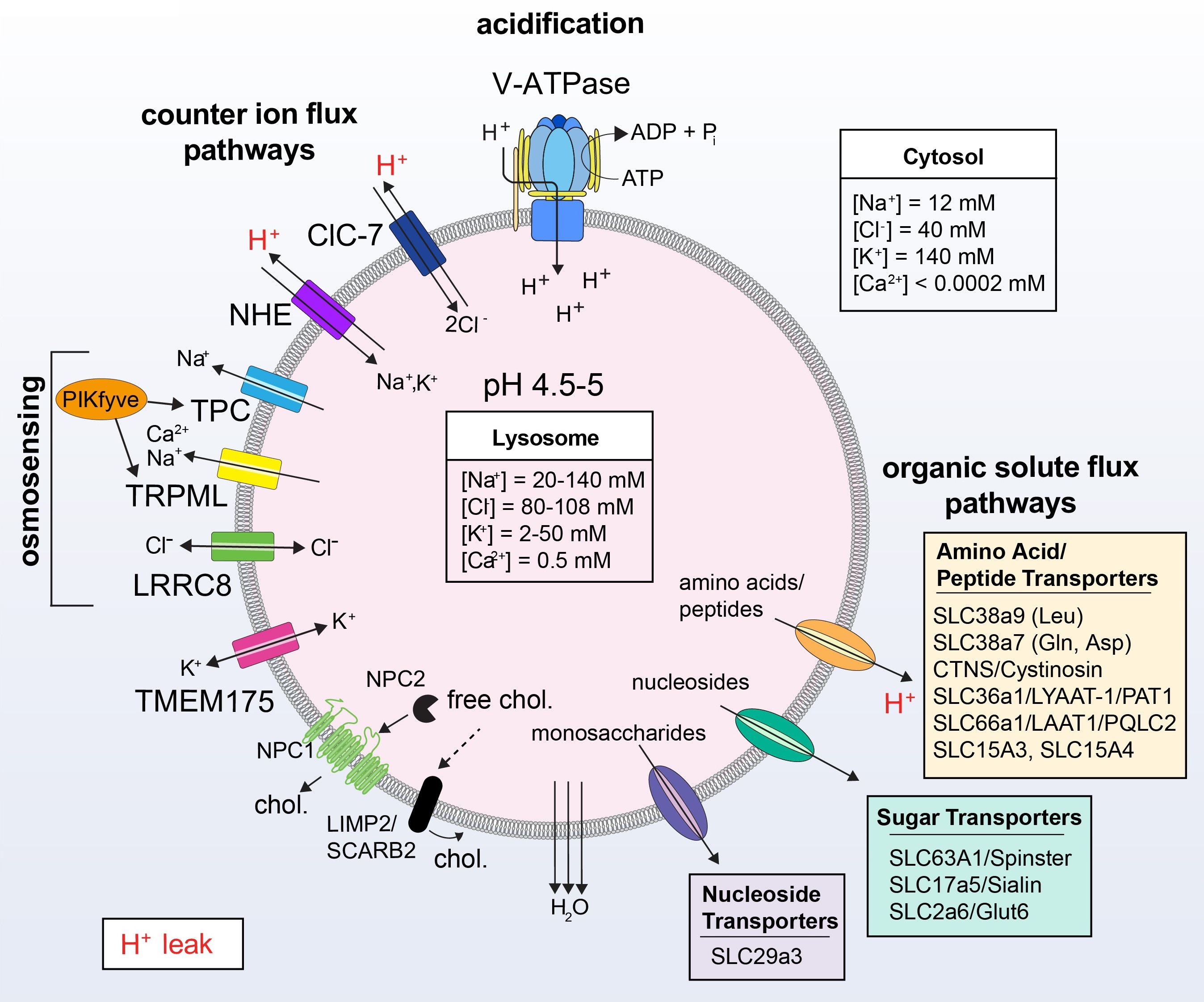
Fig. 3. Ionic and organic solute fluxes of lysosomes. The steady-state pH of lysosomes is maintained by the rate of H+ influx (mediated by the V-ATPase), the rate of H+ efflux or 'leak', and the conductance of counter-ions. At a pH of 4-5, lysosomal [H+]lumen can be 1000-fold higher than [H+]cytosol, which is at pH 7.2. The pump can work against this gradient and does not acidify the lysosome further, partly because of the H+ leak which is closely tied to the activity of solute transporters. These transporters, some of which are dependent on either the H+ or Na+ gradient to function, mediate the efflux of newly liberated amino acids, nucleosides, and monosaccharides derived from incoming endocytic cargo. NHEs functioning at the lysosome utilize the H+ gradient and exchange monovalent cations (e.g. Na+) into the lumen. As a result, to avoid their accumulation, monovalent cations are expelled from the lysosome via channels including TPC1-2, TRPML1-3, and TMEM175, a bidirectional K+ channel. These channels are gated by PtdIns(3,5)P2 and at least in the case of TRPML2, are activated by osmotic pressure and high endomembrane tension. PIKfyve is also stimulated by osmotic stress, suggesting multiple means for osmosensing by lysosomes. To rapidly acidify earlier endocytic compartments, ClC-7 provides a counter-ion force in the form of Cl- influx. While ClC-7 is another source of the H+ leak, the net negative charge of the lumen supports the activity of the V-ATPase from an electrical standpoint. Cl- efflux, on the other hand, involves LRRC8 and other putative transporters/co-transporters. Like TRPML2, LRRC8 also senses the ionic strength of the cytosol. Importantly, monovalent ion and solutes flux dictate hydrostatic pressure and membrane tension on the lysosome and ultimately fission/traffic.